Ad All The Learning Tools You Need In One Place. To write a balanced chemical equation first write the skeleton equations.

How To Balance Chemical Equations 11 Steps With Pictures
Fe3O4 Commercially it is made by for example redung Fe2O3 with CO 3Fe2O3 CO.

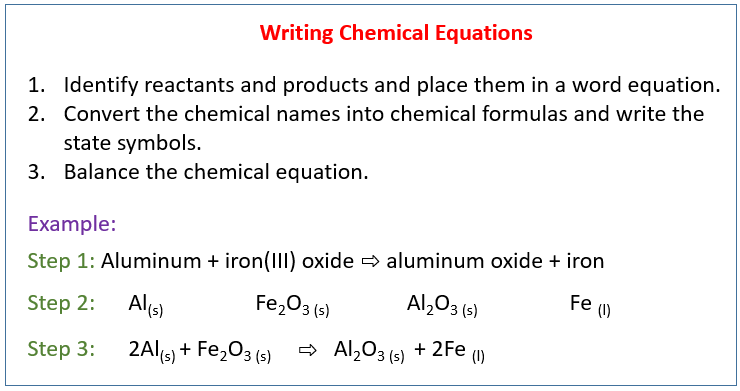
. So this question asked us to explain the steps of balancing chemical equations so the first step is to write the unbalanced equation. Identify reactants and products and place them in a word equation. Writing a balanced chemical equation lessons examples and solutions write equations for the following reactions studyrankers reaction shown here balance s lesson transcript study com identify type of in each case cbse class 10 science learn forum apling learning chegg 3 steps balancing process photosynthesis given physical states all substances.
Write the names of the products to the right of the arrow also separated by plus signs. N2 O2 N2O5 unbalanced N 2 O 2 N 2 O 5 unbalanced Next count the number of each type of atom present in the unbalanced equation. Place these in the proper order.
Write a balanced equation for the reaction of molecular nitrogen N 2 and oxygen O 2 to form dinitrogen pentoxide. The balanced chemical equation for the combustion of glucose in the laboratory or in the brain is as follows. Correctly order the steps necessary to balance a chemical equation.
However Li dropped his note cards so they are now out of order. Do a final check to make sure the equation is balanced. C 6H 12O 6s 6O 2g 6CO 2g 6H 2Ol Construct a table showing how to interpret the information in this equation in terms of.
We Have All The Tools You Need In One Place. To write a balanced chemical equation first write the skeleton equation. In order to balance the chemical equation you need to make sure the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
1 determine the correct formulas for all the reactants and products. The insoluble residue contains the impurities of silica and iron oxide. Solution First write the unbalanced equation.
Write a balanced chemical equation including the state symbols. Write an unbalanced equation 2. The cards describe the steps required to calculate percent yield.
Calculate the theoretical yield of the reaction. Start Earning Grades Today. In both processes is washed dried and heated ignited to get Al 2.
Balance the atoms of each element in turn by adding coefficients before the chemical formulas of reactants and product-compounds first elements last. 2 The filtrate is warmed and neutralised by passing carbon dioxide through the solution to precipitate aluminium hydroxide. Place the steps in the correct order by entering the numbers 1 through 5 next to the correct steps below.
So the element by element comparison includes counting elements and then appropriately adding coefficients. An old way of writing it was FeOFe2O3 The balanced equation for production from the elements is - 3Fe 2O2 -. Then use coefficients to balance the equation so that it obeys the law of conservation of mass Write a skeleton equation for heating copper II sulfide in the presence of diatomic oxygen produces pure copper and sulfur dioxide gas.
A single molecule of glucose. 2 write skeleton equation by placing the formulas for the reactants on the left and the formulas for the products on the right with the yields sign in between. Convert the chemical names into chemical formulas.
Write a word equation. What are the steps for writing and balancing a chemical equations. Write a balanced chemical equation.
Then use a coefficients to balance the equations so that the same number of each atom appears on both sides of the equations. Notice that no plus sign is needed on the product side of this equation because ironIII oxide is the only product. Steps for Writing Balanced Chemical Equations STUDY Flashcards Learn Write Spell Test PLAY Match Gravity step 1 Click card to see definition identify the reactants and products.
CuS O2 heat - Cu and SO2. Place the steps necessary to balance a chemical equation correctly in order starting with the first step at the top of the list. Hydrogen gas Oxygen gas Water Click again to see term step 2 Click card to see definition.
If you simply verify or check final equation to make sure that. And then once you finished the final step once youve put all the coefficient and you think you counted each element by one by one. To write a word equation write the names of the reactants to the left of the arrow separated by plus signs.
The fused mass is treated with water. Place reactants on the left of the reaction arrow and products on the right. In order make both sides equal you will need to multiply the number of atoms in each element until both sides are equal.
Sodium hydroxide ironII chloride sodium chloride ironII hydroxide. Place them based on the chemical equation and write the state symbols. If two or more reactants or products are involved separate their formulas with plus signs.
So what this means is that you just need to write all of the reactant and the products but you dont need to include their coefficients which means that once again its not yet balance. 3 The precipitate obtained of AlOH 3. The idea is not balanced and your balance that I just put a three in front of their.
Adjust the coefficients such that there Question.

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab
Writing A Balanced Chemical Equation Video Lessons Examples And Solutions

Balancing Chemical Equations Lesson Plan A Complete Science Lesson Using The 5e Method Of Instructi Chemical Equation Equations Balancing Equations Chemistry
0 Comments